Trace Element Analysis
using
Photon Acivation Analysis
at
Idaho Accelerator Center
Photon Activation Analysis (PAA) is a method of detecting and quantifying the elemental and isotopic composition of samples.
Advantages:
- Sensitive to a large variety of elements in a single measurement, often at the parts per million or parts per billion range.
- Probes the bulk composition of the material.
- Relatively nondestructive. Appropriate for archeological objects, works of art, forensic evidence, or scientific samples for which other tests need to be run.
- Minimal or often no sample preparation.
- Provides isotopic as well as elemental composition.
- Typical turn around times on the order of one day.
The technique in a nutshell:
- An electron beam in the 10’s of MeV energy region impinges upon a metal radiator.
- A continuous spectrum of bremsstrahlung photons is generated.
- These photons impinge upon the sample to be studied – either solid, liquid, or gas.
- A small fraction of the nuclides in the sample undergo nuclear reactions – proton knockout, neutron knockout, proton and neutron knockout, two proton knockout, two neutron knockout, and photon absorption to an excited state (isomer).
- The products of these reactions emit photons of characteristic energies over time. These photons are detected in a detector with very high energy resolution (a high purity germanium detector) to reveal the precise composition of the sample.
Some examples:
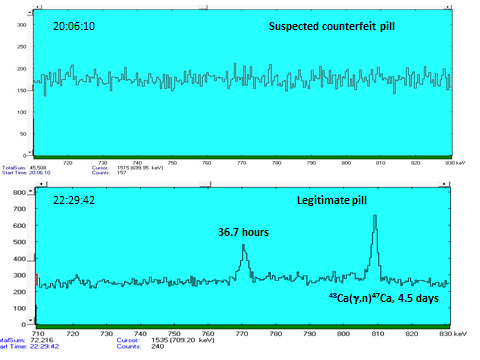
- Calcium (example from counterfeit drug detection)
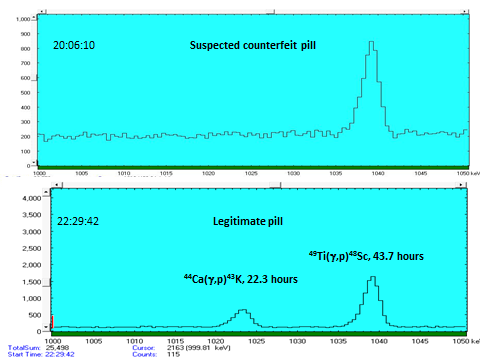
- Titanium (also from counterfeit drug detection studies)
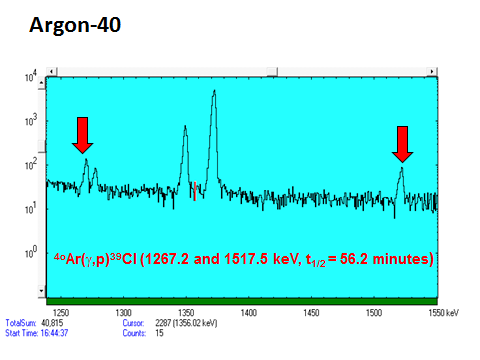
- Argon
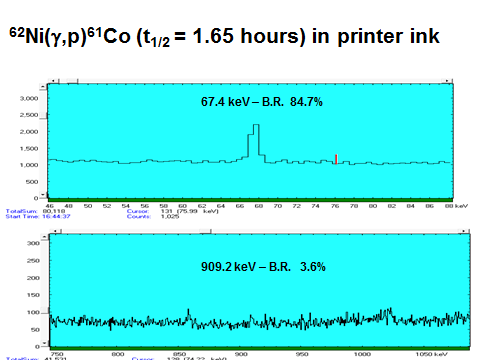
- Nickel (detected in HP 920 printer ink)
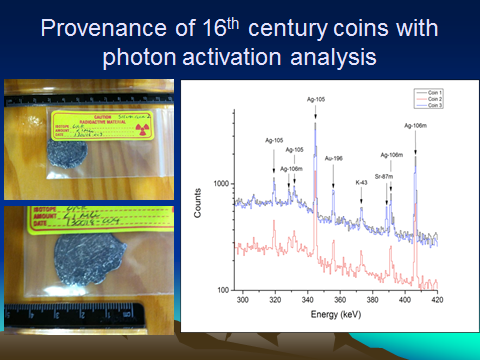
- Some 16th century coins recovered from a shipwreck
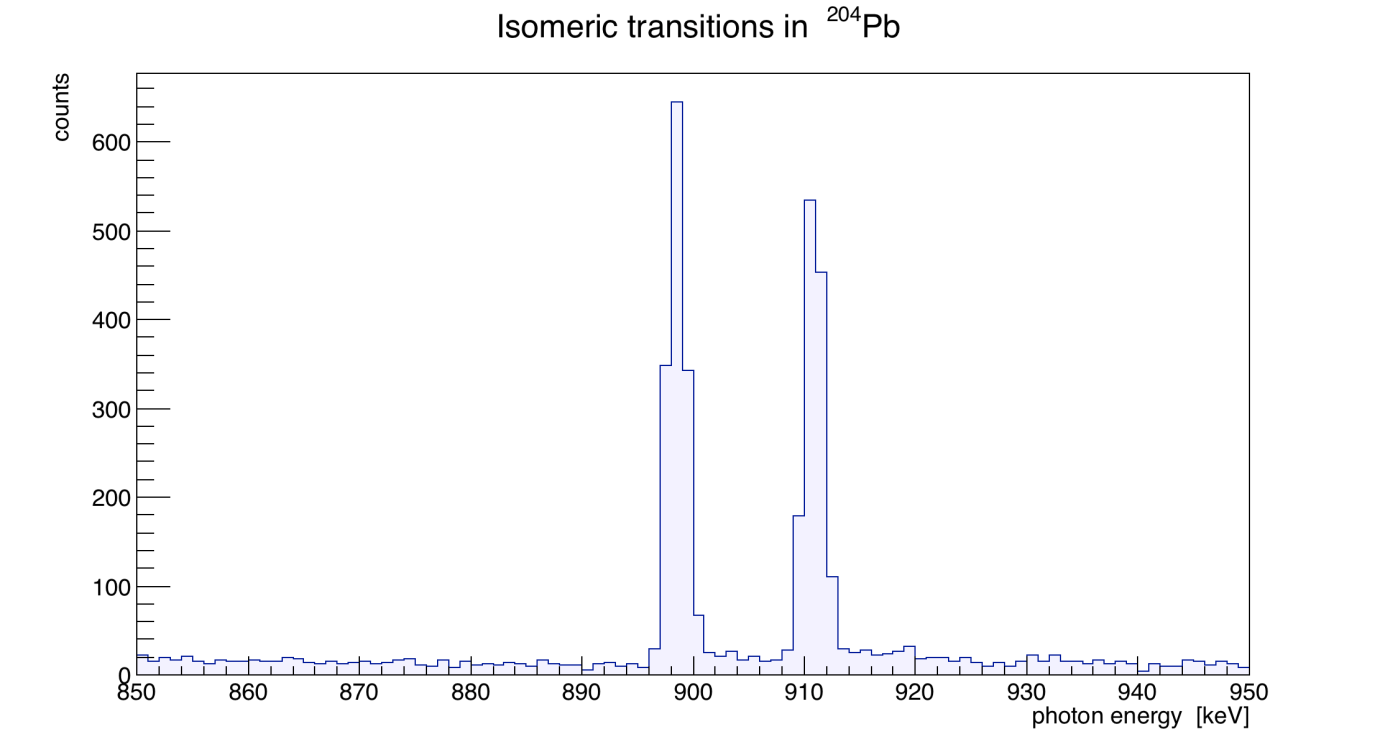
- Lead (from lead isotope fingerprinting studies)